RXFastFind: Your Quick Medication and Supplement Reference - Page 2
Digital Adherence Tools for Generic Medications: A 2026 Guide
Explore digital tools that help patients stick to generic meds. Learn about cost-effective solutions, real-world data, and practical steps to improve adherence. Cutting-edge tech is reducing healthcare costs by $300 billion annually.
How Governments Control Generic Drug Prices Without Direct Price Caps
Government doesn't set prices for generic drugs - it builds competition. Learn how FDA approvals, FTC enforcement, and market forces keep generics affordable without price controls.
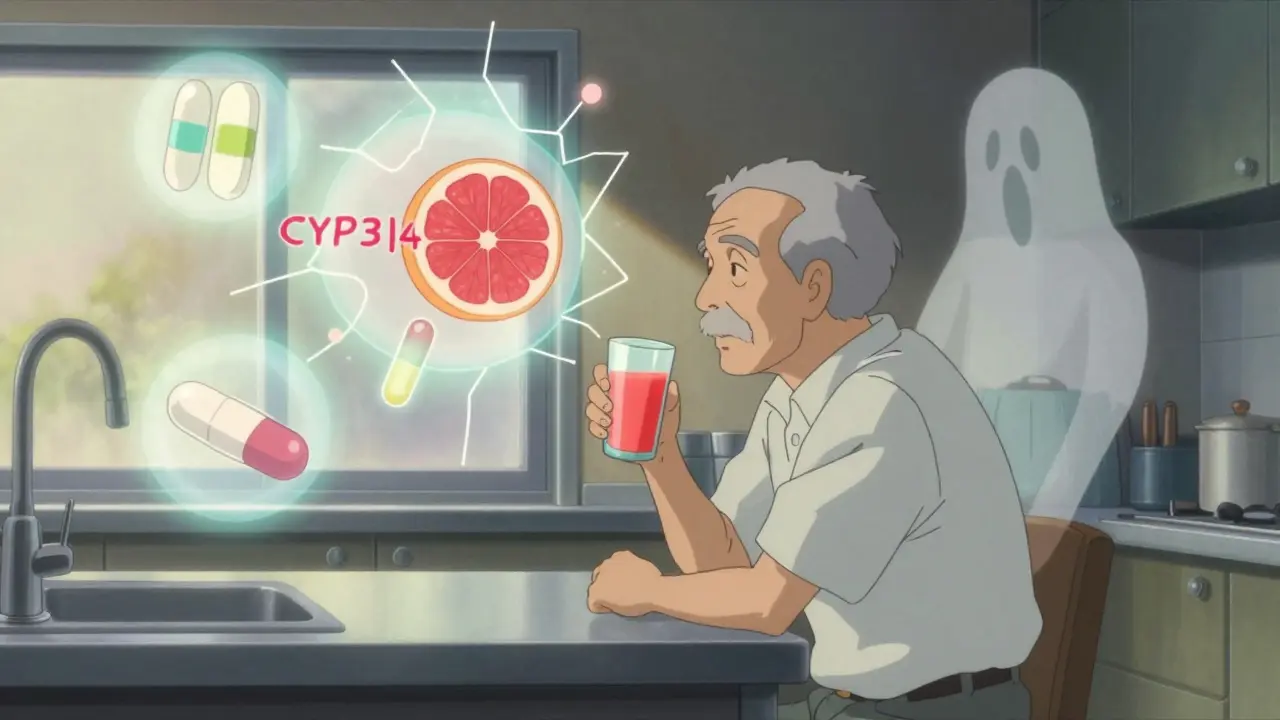
Grapefruit Juice and Medications: What You Need to Know Before You Drink
Grapefruit juice can dangerously increase levels of many common medications, leading to serious side effects. Learn which drugs are risky, why the interaction lasts days, and how to stay safe.
Thyroid Medications: Levothyroxine Safety and Monitoring
Levothyroxine is effective for hypothyroidism, but safety depends on proper dosing and regular TSH monitoring. Learn how to avoid common mistakes, recognize dangerous side effects, and ensure your treatment stays on track.
Compounded Medications: When Custom Formulas Are Needed for Personalized Care
Compounded medications offer custom drug formulas for patients who can't use standard pills due to allergies, dosage needs, or swallowing issues. But they come with risks-here's how to use them safely.
Measuring Education Effectiveness: Tracking Generic Understanding in Patient Care
Effective patient education isn't about handing out brochures-it's about knowing if patients truly understand how to manage their health. Learn how direct observation, teach-back, and rubrics track real understanding-not just recall.
Quality concerns: when clinicians question generic manufacturing
Clinicians are raising alarms about generic drug quality, especially when manufactured overseas. Studies show higher adverse event rates for generics made in India compared to U.S.-made versions. Transparency, inspection reform, and domestic production may be key to fixing the system.
Air Travel With Ear Problems: Equalization and Safety Tips
Learn how to prevent ear pain during flights with proven equalization techniques, safe decongestant use, and smart tips for kids and frequent travelers. Avoid airplane ear with simple, science-backed strategies.
Ketamine and Esketamine for Depression: What You Need to Know in 2026
Ketamine and esketamine offer rapid relief for treatment-resistant depression. Learn how they differ in effectiveness, side effects, cost, and access-and which one might be right for you in 2026.
How to Report Side Effects and Adverse Drug Reactions to the FDA Using MedWatch
Learn how to report side effects and adverse reactions to the FDA using MedWatch. Step-by-step guide for patients, caregivers, and healthcare providers on filling out forms, understanding what to report, and why your report matters.