Every year, Americans save billions of dollars by choosing generic drugs instead of brand-name ones. In fact, generic drugs make up 90% of all prescriptions filled in the U.S. But with so many options-and so many fake ones out there-how do you know you’re getting the real thing? You don’t need a pharmacy degree to spot a legitimate generic drug. You just need to know what to look for.
What Makes a Generic Drug Legitimate?
A legitimate generic drug isn’t a copy. It’s not a knockoff. It’s a medically identical version of a brand-name drug, approved by the FDA after proving it works the same way in your body. The active ingredient? Exactly the same. The dose? Exactly the same. The way your body absorbs it? Within 80-125% of the brand-name version-that’s the FDA’s strict bioequivalence standard. The FDA doesn’t approve generics lightly. Every generic manufacturer must pass the same rigorous inspections as brand-name companies. In 2022, the FDA inspected over 2,500 generic drug facilities. They tested more than 1,000 samples. And 98.4% of generic applications met quality standards on the first try. Here’s the truth: if you buy a generic drug from a licensed U.S. pharmacy, it’s just as safe and effective as the brand. But if you buy it from a shady website, a street vendor, or an unmarked bag at a gas station? That’s a different story.What Legitimate Generics Look Like (And What Doesn’t)
You might notice your generic pill looks different from the brand. Maybe it’s a different color. Maybe it’s round instead of oval. Maybe it says “TEVA” instead of “Lipitor.” That’s normal. Federal law says generic manufacturers can’t copy the exact look of brand-name drugs to avoid trademark issues. So appearance changes are expected. But here’s what should never change:- Imprint code: Every pill has a unique number or letter stamp-like “100” or “L484.” If it’s missing, smudged, or looks hand-written, walk away.
- Consistency: Legitimate pills are factory-made. They all look the same. No cracks, no bubbles, no crumbling edges.
- Texture: If it feels powdery, sticky, or too hard, something’s wrong. Counterfeit pills often use cheap fillers that don’t hold together.
- Smell: Some pills have a faint chemical odor. But if it smells like plastic, rot, or something off, it’s not right.
Check the Packaging Like a Pro
The container matters just as much as the pill. Legitimate generics come in sealed, tamper-evident packaging with clear, professional labeling. Look for:- Your name and the prescriber’s name
- The drug name and strength (e.g., “Atorvastatin 20 mg”)
- The manufacturer’s name (Teva, Sandoz, Mylan, etc.)
- A lot number and expiration date
- Barcodes and FDA-mandated warning labels

Where to Buy Generic Drugs (And Where to Avoid)
You’re safest buying generics from:- Local, licensed pharmacies (CVS, Walgreens, Rite Aid, independent pharmacies)
- Online pharmacies with the .pharmacy seal (verified by NABP)
- Hospital or clinic pharmacies
- Websites that sell drugs without a prescription
- Online stores with names like “GlobalPharmaDeals” or “CheapMedsUSA”
- International sellers shipping from countries with weak regulations
- Social media ads offering “discounted” generics
Verify Your Drug with Free Tools
You don’t need to guess. Use these free tools to double-check:- FDA’s Orange Book: Search by drug name to see all approved generics and their manufacturers. You’ll find the exact product codes and therapeutic equivalence ratings.
- NABP’s .pharmacy Checker: Go to nabp.pharmacy and enter the website name. If it’s not listed, don’t buy.
- FDA’s Drug Recall Database: Type in the lot number from your bottle. If it’s been recalled, you’ll know.
- MediSafe App: Scan the 2D barcode on newer generic bottles. The app verifies authenticity in seconds.
What to Do If Something Feels Off
You notice your blood pressure meds aren’t working like before. Or your generic antidepressant gives you a weird aftertaste. Or the pills look different than your last refill. Don’t ignore it. Stop taking it. Call your pharmacist. Ask them to check the lot number and manufacturer. If they’re unsure, ask for the brand-name version while you figure it out. Then report it. The FDA’s MedWatch program takes reports from consumers. In 2022, they received over 1,200 reports of counterfeit drugs. 41% involved heart medications. 29% were for erectile dysfunction pills. Many people didn’t realize they’d been sold fakes until their symptoms changed. Reporting saves lives. One fake pill can kill. And if you don’t report it, someone else might take it.
Why Generic Drugs Are Safe-When You Get Them Right
The truth? Generic drugs work. A 2021 JAMA study looked at over 2,000 FDA-approved generics. 98.7% matched the brand-name drug’s absorption rate within 1%-that’s better than most people expect. People on generic statins, diabetes meds, and blood pressure drugs report the same results as those on brand names. The difference? Cost. A 30-day supply of brand-name Lipitor might cost $300. The generic? $12. That’s not a compromise. That’s smart healthcare. But that savings only matters if you’re getting the real thing. Fake drugs don’t just waste money-they can poison you. Some counterfeit pills contain rat poison, fentanyl, or no active ingredient at all.Final Checklist: Your 5-Second Generic Drug Safety Test
Before you leave the pharmacy, do this quick check:- Is the pharmacy licensed? (Look for the state license displayed or check nabp.pharmacy)
- Does the pill have a clear, consistent imprint? (No smudges, no missing letters)
- Is the label professional? (No typos, no foreign text, no hand-written notes)
- Is the bottle sealed and tamper-evident? (No broken seals, no loose caps)
- Does the lot number match what’s on the FDA’s recall site? (Ask your pharmacist to check)
Generic drugs are one of the safest, most cost-effective parts of modern medicine. But only if you get them from the right place. Don’t let a bad choice cost you more than money.
Can generic drugs be less effective than brand-name drugs?
No, not if they’re FDA-approved. Legitimate generics must prove they work the same way in your body as the brand-name drug. The FDA requires them to match the brand’s absorption rate within 80-125%. Studies show 98.7% of approved generics meet this standard with near-identical results. If your generic isn’t working, it’s likely not the drug-it’s the source. Counterfeit or improperly stored pills can fail, but FDA-approved generics from licensed pharmacies are just as effective.
Why do generic pills look different from brand-name ones?
Federal law prohibits generic manufacturers from copying the exact appearance of brand-name drugs to avoid trademark infringement. That’s why generics often have different colors, shapes, or markings. But the active ingredient, dose, and how your body absorbs it must be identical. The difference is only cosmetic. If the pill’s imprint, texture, or packaging looks off, that’s a red flag-not the color.
Is it safe to buy generic drugs online?
Only if the website is verified. Look for the .pharmacy seal, which means the site is accredited by the National Association of Boards of Pharmacy. Over 96% of online pharmacies selling drugs without a prescription are illegal. Sites that offer “discounted” generics without a prescription, ship from overseas, or don’t list a physical address are almost always selling counterfeit drugs. Stick to verified pharmacies or your local pharmacy’s online portal.
How can I tell if my generic drug has been recalled?
Check the lot number on your bottle against the FDA’s Drug Recall Database. You can search by drug name, manufacturer, or lot number at fda.gov/safety/recalls. If your drug is listed, stop taking it immediately and return it to the pharmacy. Pharmacists are required to notify you if your medication is recalled, but it’s smart to check yourself-especially if you notice changes in how the drug works or looks.
What should I do if I suspect I received a fake generic drug?
Stop taking the medication immediately. Contact your pharmacist and ask them to verify the source and lot number. Then report it to the FDA’s MedWatch program at fda.gov/medwatch or by calling 1-800-FDA-1088. Include the drug name, lot number, where you bought it, and any symptoms you’ve noticed. Your report helps the FDA track counterfeit drugs and protect others. Don’t wait-fake drugs can be dangerous or even deadly.
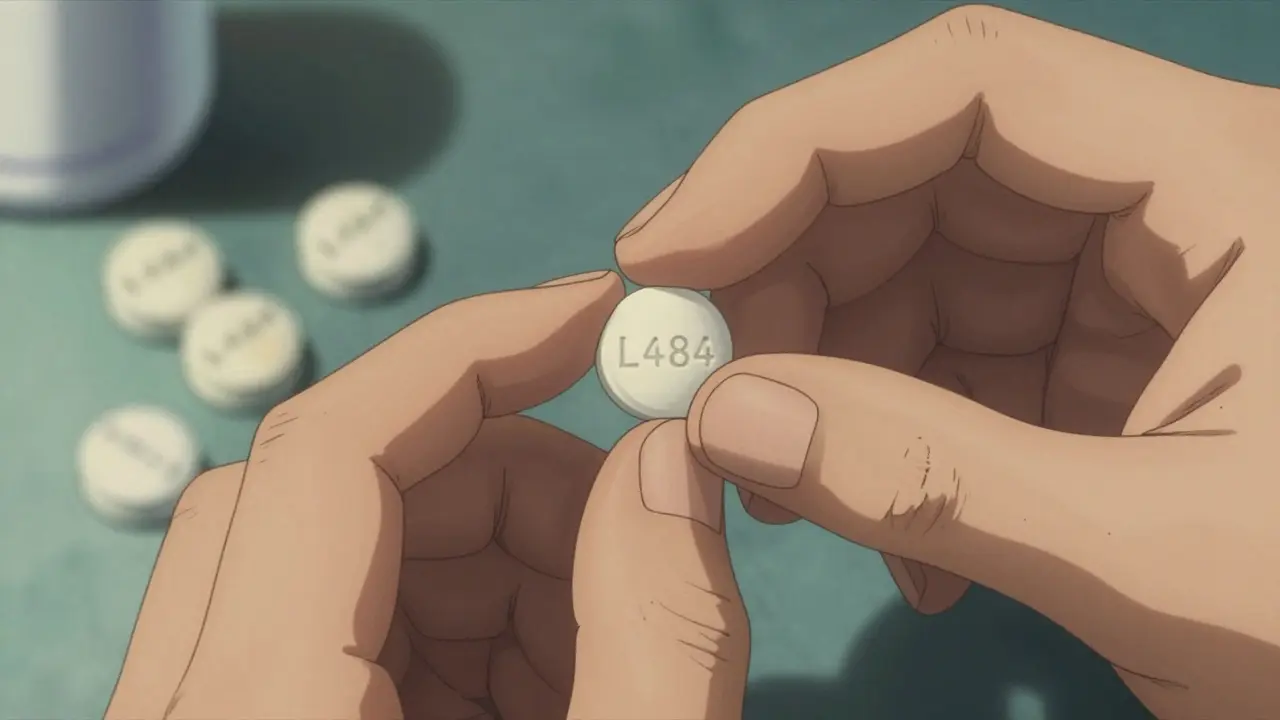
Hayley Ash
So let me get this straight - if I buy a pill that looks like a rainbow exploded on it and has ‘TEVA’ stamped on it, I’m somehow safer than if I bought the brand name that looks like a corporate logo vomited on a white tablet? Yeah right. I once got a generic for anxiety that tasted like burnt plastic and made me feel like my brain was being slowly digested by a sloth. FDA says it’s ‘bioequivalent’ - great. My body says it’s a betrayal.
And don’t even get me started on the ‘.pharmacy’ seal. That’s like trusting a guy who says he’s a cop because his badge says ‘COP’ in Sharpie.
kelly tracy
You people are naive. The FDA doesn’t regulate foreign manufacturers. Most generics come from India and China where inspectors get paid in rice and bribes. That ‘98.4% approval rate’? That’s the same number they used for the tainted heparin that killed 80 people in 2008. They’re not testing the pills - they’re testing the paperwork.
I’ve seen pills with the wrong imprint code. I’ve seen bottles with no lot numbers. I’ve seen pharmacists shrug and say ‘it’s FDA approved’ like that’s a magic shield. It’s not. It’s a loophole with a seal.
srishti Jain
Generic drugs work fine if you get them right. But most people don’t check. They just take whatever the pharmacy hands them. Big pharma doesn’t care. They just want you to keep buying. If you’re lucky, you get the real thing. If not? You get a placebo with side effects.
Stop trusting labels. Start checking the imprint. Always.
Cheyenne Sims
The assertion that generic drugs are equivalent to brand-name medications is misleading without proper context. While the FDA mandates bioequivalence within an 80-125% range, this allows for clinically significant variability in pharmacokinetics. Patients with narrow therapeutic indices - such as those on warfarin, levothyroxine, or phenytoin - may experience adverse outcomes due to inter-generic switching. Regulatory compliance does not equate to clinical safety for all populations.
Furthermore, the reliance on visual inspection of pill imprint codes is insufficient. Counterfeiters now replicate these with laser precision. Verification must include third-party laboratory testing, which is not feasible for the average consumer.
Kelly Gerrard
Thank you for this guide. It’s so important to know what to look for. I’ve been on generics for years and never thought to check the imprint or lot number. I’m going to start doing this now. Your checklist is clear and practical.
People need to stop assuming all pills are the same. Your health isn’t a gamble.
Glendon Cone
Big respect for breaking this down 👏
I work in a clinic and see people panic because their generic pill looks different - turns out they just switched from Mylan to Teva and freaked out. The key is education. Most people don’t realize the color change is legal and intentional. But you’re right - if the imprint is missing or the pill crumbles? That’s not normal.
Also - never buy from Instagram ads. I’ve seen guys buy ‘Viagra generics’ from a guy named ‘Mike’ who lives in a van. One guy ended up in the ER with kidney failure. It was laced with fentanyl.
Use the FDA database. It’s free. It’s real. It saves lives.
Henry Ward
Oh please. You’re all acting like this is some groundbreaking revelation. The FDA is a corporate puppet. They approve generics from factories that have been shut down three times. The only reason they ‘pass’ 98% is because they don’t test enough. And you think a pill with a stamp is safe? I’ve seen fake pills with perfect imprints - printed with laser engraving from China.
You’re not safe. You’re just lucky. And when your heart gives out because your ‘generic’ blood thinner had zero active ingredient, don’t come crying to me.
And for the love of god - stop using the word ‘bioequivalent’ like it’s a blessing. It’s a loophole.
Aayush Khandelwal
Let’s not conflate regulatory compliance with operational integrity. The FDA’s bioequivalence threshold of 80-125% AUC and Cmax is statistically valid but pharmacodynamically porous - especially for CYP450-metabolized agents like SSRIs or anticoagulants. Add to that the supply chain opacity of APIs sourced from Tier-3 manufacturers, and you’ve got a perfect storm for subtherapeutic exposure.
That said - if you’re procuring from a licensed U.S. pharmacy with a verifiable lot trace, the risk profile is negligible. But most consumers don’t even know what an API is, let alone how to cross-check a manufacturer’s GMP certification.
TL;DR: Trust the system only if you verify the source. Otherwise, you’re gambling with your cytochrome P450 enzymes.