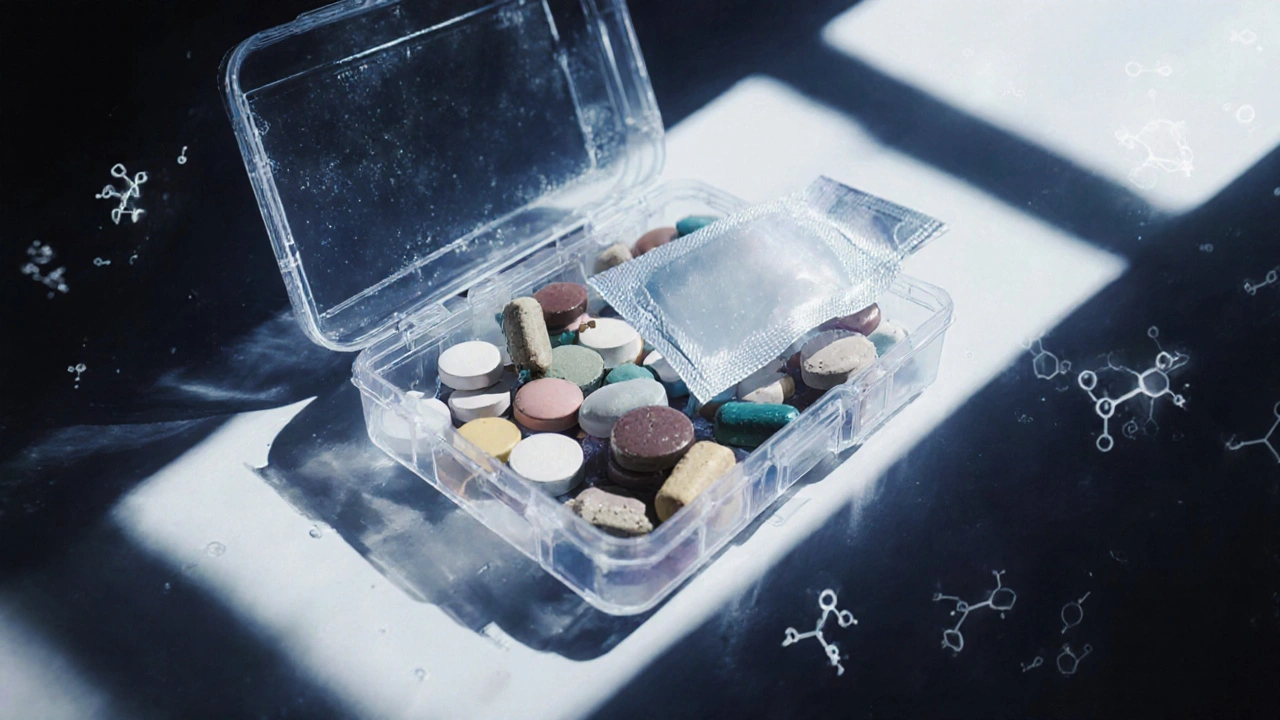
When you take your meds out of the original bottle and put them into a pillbox or a pharmacy repackaged vial, you’re changing their environment. And that change can make them less effective-or even unsafe. The expiration date on the bottle? It doesn’t apply anymore. That’s not a myth. It’s a fact backed by the FDA, USP, and real-world studies. If you’re using a weekly pill organizer or getting meds repackaged by your pharmacy, you need to know how to tell if they’re still good.
Why Original Expiration Dates Don’t Work for Repackaged Meds
The expiration date on your prescription bottle is based on the drug’s stability in its original container-the one designed by the manufacturer. That container has special barriers: moisture-proof plastic, desiccants, light-blocking materials, and tight seals. When you transfer the pills to a plastic vial from the pharmacy or toss them into a plastic pillbox, you remove those protections. A 2019 study in the Journal of Pharmaceutical Sciences found that albuterol sulfate tablets lost 15.7% of their potency after 90 days in a standard pharmacy vial, while the same pills in their original HDPE bottle with desiccant lost only 3.2%. That’s more than a 5x difference. And albuterol isn’t even the most sensitive drug. Some meds degrade even faster. The FDA’s 2023 warning letter to a major pharmacy chain made it clear: assigning the original expiration date to repackaged meds is a violation. The agency shut down their repackaging operations for 45 days because they didn’t have written procedures to determine new expiration dates. This isn’t just paperwork-it’s patient safety.What Makes Repackaged Medications Unstable?
Three main enemies attack repackaged drugs: moisture, light, and air. Moisture is the biggest threat. Many pills, like amoxicillin, atenolol, or levothyroxine, are hygroscopic-they soak up water like a sponge. Once they do, they can break down chemically. A standard pharmacy vial lets in 0.35-0.50 grams of water vapor per square meter per day. The original manufacturer bottle? Only 0.10-0.25. That’s nearly double the moisture exposure. In humid climates like Seattle, this becomes even worse. Light degrades drugs like nifedipine, nitroglycerin, or riboflavin. Amber vials help, but most pillboxes are clear plastic. Even indirect sunlight on your kitchen counter can cause damage. A 2022 study showed nifedipine in clear pill organizers lost 12% potency in just 30 days. Oxygen causes oxidation. Drugs like aspirin, ibuprofen, and certain antidepressants slowly break down when exposed to air over time. This isn’t visible, but it’s measurable. HPLC testing can detect degradation products as small as 0.05%-far below what your eyes can see.How Long Are Repackaged Meds Actually Good For?
There’s no one-size-fits-all answer. But experts agree on time limits based on drug type and storage:- 30 days max for hygroscopic drugs: amoxicillin, doxycycline, levothyroxine, and other moisture-sensitive pills.
- 60 days max for light-sensitive drugs: nifedipine, chlorpromazine, isotretinoin.
- 90 days max for stable drugs: atenolol, lisinopril, metformin, simvastatin-when stored in amber vials with desiccant.
- Never exceed 6 months, even under ideal conditions. The International Pharmaceutical Federation (FIP) says this is the absolute outer limit.

What About Pillboxes? They’re Riskier Than You Think
Pillboxes are the wild west of medication stability. You’re mixing drugs from different manufacturers, different formulations, different coatings-all in one plastic container. No one tested that combination. A 2022 American Pharmacists Association study found that 18.7% of pillboxes with multiple medications showed physical changes within two weeks: pills sticking together, color changes, powders clumping. These aren’t just cosmetic. When drugs interact physically, they can change how they dissolve in your body-or even create toxic byproducts. And here’s the kicker: most pillboxes don’t have desiccants. Even if they did, they’re not sealed. Moisture and air get in every time you open it. That’s why experts recommend using pillboxes only for short-term use-like a 7-day supply-and never for long-term storage.How to Actually Test Stability (Without a Lab)
You don’t need an HPLC machine to protect yourself. Here’s what you can do:- Check for visible changes. Look for discoloration, crumbling, sticking, or unusual odors. If a pill looks different, don’t take it.
- Use desiccant packs. If your pharmacy doesn’t include them, ask for them. Or buy silica gel packs (the kind that come with electronics) and add one to your vial. A 2023 trial showed this extended stability by 47%.
- Store in cool, dark, dry places. Not the bathroom. Not the kitchen window. A bedroom drawer or a cabinet away from the stove is best.
- Label everything. Write the repackaging date and the new expiration date clearly. Don’t rely on memory.
- Use the 30-day rule for safety. If you’re unsure, toss it after 30 days. It’s cheaper than a hospital visit.

What Pharmacies Should Be Doing (But Often Aren’t)
The Institute for Safe Medication Practices found that 32% of community pharmacies have no formal stability protocols. That’s alarming. Pharmacies repackaging meds should:- Use amber vials for light-sensitive drugs.
- Include desiccant in every moisture-sensitive container.
- Assign a new expiration date based on drug class-not the original bottle.
- Keep records of repackaging dates and expiration dates.
- Train staff on stability risks.
What You Can Do Right Now
If you’re using repackaged meds or a pillbox:- Ask your pharmacist: “What’s the new expiration date for these pills?” If they say “the same as the bottle,” walk out and find another pharmacy.
- Request amber vials and desiccant packs for moisture- or light-sensitive drugs.
- Use pillboxes only for 7-14 days, not months.
- Keep a log: when you repackaged, what the new expiration is, and when you threw it out.
- If you’re unsure, throw it out. Medication waste is a small cost compared to ineffective or dangerous meds.
What’s Coming Next
The USP is finalizing General Chapter <1790> by December 2024. It will standardize testing: minimum 12 samples per time point, at least 3 testing intervals for short-term meds. The FDA is also pushing for vacuum decay testing to detect tiny leaks in packaging. But until then, the responsibility falls on you and your pharmacist. Don’t assume. Don’t guess. Ask questions. Document. Protect yourself.Medications are powerful tools. But they’re not magic. They degrade. They react. They fail. And if you’re not treating repackaged meds with the same care as the original, you’re risking your health.
Can I use the original expiration date on repackaged medications?
No. The expiration date on the original bottle only applies if the medication stays in its original container with the original closure and desiccant. Once you transfer it to a pillbox or pharmacy vial, the stability changes. The FDA explicitly states that pharmacies must establish new expiration dates for repackaged products. Using the original date is a violation of federal guidelines and puts your health at risk.
How long can I safely store meds in a pillbox?
For safety, limit pillbox use to 7-14 days. Most pillboxes are clear plastic, unsealed, and lack desiccants. A 2022 study found that 18.7% of multi-drug pillboxes showed physical changes like caking or discoloration within two weeks. Even if pills look fine, their potency may be dropping. Use pillboxes only for short-term organization, not long-term storage.
Which medications are most at risk when repackaged?
Hygroscopic drugs like amoxicillin, doxycycline, and levothyroxine absorb moisture quickly and degrade within 30 days. Light-sensitive drugs like nifedipine, nitroglycerin, and riboflavin break down under even indirect light. Drugs with narrow therapeutic indexes-like warfarin, digoxin, or lithium-are especially dangerous if potency changes, because small drops can cause treatment failure or toxicity.
Can I add desiccant packs to my repackaged meds?
Yes, and you should. Silica gel desiccant packs-like those found in shoeboxes or electronics packaging-can extend stability by up to 47%, according to a 2023 multicenter trial. Ask your pharmacist to include them, or add a small pack yourself. Just make sure it doesn’t come into direct contact with the pills. Place it on the side or under the lid.
What should I do if my pharmacy won’t give me a new expiration date?
If your pharmacy refuses to assign a new expiration date, ask for the original bottle back. If they won’t give it to you, consider switching pharmacies. You have the right to know how long your meds are safe. Pharmacies that skip this step are cutting corners on safety. The FDA has issued warning letters and shutdowns for exactly this issue. Don’t risk your health for convenience.
Alyssa Torres
OMG I had no idea my pillbox was basically a chemical hazard zone 😱 I’ve been using mine for months with my thyroid meds and nifedipine… I’m tossing it today and getting amber vials. Thank you for this life-saving post.
Summer Joy
THIS IS WHY WE CAN’T HAVE NICE THINGS. Pharmacies are just profit-driven garbage factories. I asked for a new expiration date and they laughed. LMAO. I’m switching to mail-order and keeping everything in original bottles. 💀
Nicole Ziegler
Just added silica gel packs to my vials. Feels like a lab scientist now 🧪✨
Kristi Bennardo
Are you seriously telling me that 41 states already enforce this and the rest are endangering lives? This isn’t negligence-it’s criminal. The FDA should be shutting down every non-compliant pharmacy in the country. People are dying because of this laziness.
Bharat Alasandi
bro i use pillbox for 2 weeks max n always write date on it. also got desiccant from amazon-$2 for 100 packs. life changed. no more panic when i see my pills look weird.
Ravi boy
in india we just use ziplock bags with a label and keep in drawer no one cares about desiccant or amber bottles but we dont have humidity like seattle so maybe its fine idk
Shiv Karan Singh
lol 90 days max? you think the FDA gives a damn? they’re the same people who let Purdue push oxycontin. this is all fearmongering to sell you more bottles. your meds are fine. trust me i read the internet.
Ron and Gill Day
Wow. What a tedious, over-researched, self-congratulatory essay. I’ve been using pillboxes for 12 years. I’m 73. I’m still alive. You think your HPLC data makes you smarter than my grandma’s intuition? Please. The real danger is over-medicalizing every damn thing.
Liam Strachan
Interesting read, thanks for sharing. I’ve always just kept meds in original bottles and used a pillbox for 7 days max-never thought about moisture levels. Might ask my pharmacist about desiccant packs next time. Always good to be cautious.
Matthew Karrs
Who’s to say the original bottle isn’t already compromised? What if the manufacturer used cheap HDPE? What if the desiccant was expired before it left the warehouse? This whole thing feels like a distraction from the real issue: Big Pharma doesn’t want you to know how fragile these drugs really are.
Aruna Urban Planner
The underlying tension here is between systemic infrastructure and individual agency. Pharmacies lack resources; patients lack awareness. The solution isn’t just labeling or desiccants-it’s policy reform, subsidized testing kits, and public health literacy campaigns. Without structural change, we’re just rearranging deck chairs on the Titanic.
Matthew Peters
Okay but what about the 2024 USP chapter? Is it gonna be mandatory? Because if it’s just a recommendation, we’re still screwed. Also-has anyone tried vacuum-sealing pillboxes? I saw a guy on TikTok do it with a FoodSaver. Kinda wild but… maybe?
Gerald Cheruiyot
Medications are tools not magic. They degrade. They react. They fail. I’ve been reading this whole thing and it’s just… true. I used to think expiration dates were marketing. Now I know they’re a last line of defense. I’m changing my habits. No more pillbox after 14 days. No more bathroom storage. Just… care.